Acreditación y certificación de calidad hospitalaria ¿diferentes o similares?
Fecha: 03/06/2016
Idioma: Castellano
Web: ver aquí
Autor: R. M. Guerra Bretaña, Y. A. Marín Álvarez
Procedencia: Revista Ingeniería Biomédica, Universidad EIA
(1) Cátedra de Calidad, Metrología y Normalización, Centro de Biomateriales, Universidad de La Habana, Cuba
(2) Grupo de Investigación COINDE, Politécnico Colombiano Jaime Isaza Cadavid, Medellín, Colombia
RESUMEN
La evaluación externa y la gestión de la calidad hospitalaria son dos aspectos íntimamente relacionados del mismo fenómeno: la necesidad de mejorar la calidad de los servicios de salud y brindar confianza de esta calidad a todas las partes interesadas. El objetivo de este estudio es analizar los programas de acreditación hospitalaria y los sistemas de gestión y certificación de calidad ISO 9001 de las instituciones sanitarias, así como identificar los beneficios de cada uno de ellos, sus similitudes y diferencias. Las acreditaciones hospitalarias y las certificaciones ISO 9001 son instrumentos importantes para mejorar la calidad del servicio de salud y para dar confianza a la sociedad sobre el proveedor de atención médica. Ambos guían las estrategias de gestión para mejorar la calidad del servicio y la seguridad del paciente. Difieren en algunos aspectos: la acreditación se basa en las mejores prácticas de la calidad de la asistencia médica y tiene un carácter más técnico. La norma ISO 9001 está más orientada al proceso y constituye un marco adecuado para incorporar los requisitos de los programas de acreditación hospitalaria y las metodologías internacionales existentes para la gestión del riesgo en las instituciones de salud.
Palabras clave: acreditación, certificación ISO 9001, calidad hospitalaria.
ABSTRACT
The external evaluation and management of hospital quality are two intimately related aspects of the same phenomenon: the need to improve the quality of health services and provide confidence of this quality to all stakeholders. The objective of this study is to analyze the hospital accreditation programs and ISO 9001 quality management and certification schemes of health institutions, as well as to identify the benefits of each of them, their similarities and differences. Hospital accreditations and ISO 9001 certifications are important instruments to improve the health service quality and to give confidence to the society about the health care provider. Both guide management strategies to improve service quality and patient safety. They differ in some aspects: accreditation is based on the best practices of the quality of the medical assistance and have a more technical character. The ISO 9001 standard is more process oriented and constitutes a suitable framework for incorporating the requirements
of hospital accreditation programs and existing international methodologies for risk management in health institutions.
Keywords : accreditation, certification ISO 9001, hospital quality.
I. INTRODUCTION
At present, activities aimed at guaranteeing and improving quality in the health sector are carried out in two closely related directions: external evaluation and internal quality management of health institutions. These trends have evolved following the changes that have occurred in the administration of health services, under the influence of the socio-economic environment.
According to Arce [1] in the 1950s and 1960s, immediately after the creation of the World Health Organization (WHO), the administration of health services focused essentially on planning. The health organization model, initiated in 1948 by the British National Health Service (NHS), was based on a governmental organization which, through the central planning instrument, identifies the needs of the population and allocates resource. In this type of organization, the health administrators are positioned at the top of the NHS.
In the 1980s, the growth of Social Security in European countries involved the splitting of the health sector into two functions: the financing function and the health care service function. This separation moved the location of the health administrators to the institutions providing the health care services. In this way the planning started to be exercised from the institutions and aimed for the efficient management of the activities involved. At this stage, the medical audit also emerges as a systematic evaluation performed by physicians, which compares the characteristics or quality of the care provided and observed with the ideal and desired quality, according to pre-established criteria and standards [2].
In the 1990s, notions of quality and responsibility in the delivery of health services were developed, as a basis for their efficacy and efficiency. In this conception, quality administration of health services is placed at the base of the system. Previously, since the 1970s, Donabedian
[3] had raised his systemic approach to hospital quality by differentiating three areas: structure, processes, and outcomes. The structure refers to the organization of the institution and the characteristics of its human, physical and financial resources. The processes correspond to the content of care and to the way that care is executed. The outcomes represent the impact achieved with care, in terms of improvements in the health and well-being of individuals, groups or populations, as well as users’ satisfaction with the services provided. In addition, this model proposes to address the quality attributes that characterize the health service in three dimensions: the human dimension, the technical dimension and the environmental dimension.
Ross et al. [4] group the characteristics of health quality in two major dimensions: technical quality, which seeks to guarantee the safety, effectiveness and usefulness of health actions, as well as timely, effective and safe care of the users of the services; and the quality perceived by the users themselves, taking into account the material, psychological, administrative and ethical conditions in which such actions are developed. This classification corresponds to what is understood by objective quality and subjective quality.
Nowadays, quality is evaluated through Hospital Accreditation models, excellence models such as the Malcolm Baldrige Quality Award of the United States and other national or regional Awards of Excellence such as the European model EFQM (European Foundation for Quality Management); or certified using ISO 9001 generic standard from the International Organization for Standardization (ISO) and its adaptations to the health sector [5]. External peer reviews are also used in some medical specialties. These same models can be used for internal self-evaluation and for quality management in the case of the ISO 9001 standard. The Ministries of Health in some countries use the Habilitation, Accreditation or Certification of Hospitals as a guarantee of compliance with minimum standards to provide the health service [6]. All different models are of great importance not only to improve the quality of services but also to secure and enhance the trust of external stakeholders such as patients, financiers and the state [7].
The objective of this study is to analyze the accreditation and ISO 9001 certification schemes of health institutions, as well as to identify the benefits of each of them, their similarities and differences.
II. METHODOLOGY
The theoretical research methods historical-logical and analysis-synthesis are used, starting with a review of the specialized literature, to know the main approaches related to the hospital quality and compare them to draw the conclusions regarding their similarities and differences.
III. RESULTS
A. CONCEPTUAL FRAMEWORK
According to the definitions of ISO/IEC 17000 [8], certification is the third-party attestation (issue of a statement, based on a decision following review, that fulfilment of specified requirements has been demonstrated) related to products, processes, systems or persons. Meanwhile, accreditation in the ISO scheme is always relative to a conformity assessment body, and it is the thirdparty attestation related to a conformity assessment body conveying formal demonstration of its competence to carry out specific conformity assessment tasks. The organizations authorized to certify compliance with the ISO 9001 standard are the certification bodies, which must be accredited by a recognized accreditation body.
Outside the ISO framework, accreditation is employed by sectors such as education and health. Specifically Hospital Accreditation is the formal statement by a recognized authority on the ability of a hospital to carry out specific tasks, according to predefined criteria. “A selfassessment and external peer assessment process used by health care organizations to accurately assess their level of performance in relation to established standards and to implement ways to continuously improve” [9].
Health care quality would be defined as “the optimal achievable result for each patient, the avoidance of physicianinduced (iatrogenic) complications, and attention to patient and family needs in a manner that is both cost effective and reasonably documented” [9]. This definition is not in contradiction with that given by the ISO 9000 standard [10], when it expresses that quality is the “degree to which a set of inherent characteristics of an object fulfils requirements”. In the case of hospital quality, the “object” would be the health service and the requirements correspond to the attributes of the hospital quality, among them: patient safety, access, opportunity, efficacy, efficiency, patient suitability and acceptability [11,12]. These attributes are explicitly set out in hospital accreditation standards.
B. HOSPITAL ACCREDITATION PROGRAMS
The Joint Commission International (JCI) Accreditation Program is one of the most widely recognized all over the world [13]. JCI is the internationalization of the Joint Commission on Accreditation of Hospitals (JCAH), founded in the USA in 1951. Since 1987 this institution has evolved towards the Joint Commission on Accreditation of Healthcare Organizations (JCAHO), extending the accreditation model to other health institutions, in addition to hospitals. JCI was established in 1994 as a division of JCAHO with the goal of facilitating accreditation services worldwide in more than 90 countries. In 2013, JCI published the 5th edition of its international accreditation standards for hospitals, which include a section for academic medical centers. In the JCI standards all patientcentered hospital activities (Section II), those related to the management of the health institution (Section III) and those linked to the hospital as an academic medical center (Section IV), are conceptualized [14]. Quality improvement and patient safety are included in Section III.
In addition to the JCAHO program in the USA, a National Integrated Accreditation of Healthcare Organizations (NIAHO) program, by Det Norske Veritas (Norway), is being developed. This program is discussed below.
In Latin America since the early 1990s, Pan American Health Organization (PAHO), together with the Latin American Federation of Hospitals (FLH), have been working on defining the Manual of Hospital Accreditation for Latin America and the Caribbean, to provide guidelines for quality development of services.
Accreditations are generally carried out by non-state nonprofit entities, made up of representatives of all sectors that make up the health system, although in some countries, such as Cuba, this activity is assumed by the Ministry of Public Health [15].
In Colombia, the Decree No. 903 of 2014 updated the Single System of Health Accreditation -SUA (Spanish acronyms) -, to strengthen the implementation of higher standards of quality in health care [16]. The SUA is one of the components of the Mandatory System for Quality Assurance in Health and has been regulated since 2002 (Resolution 1774), and subsequently modified by Resolutions 1445 of 2006 and 123 of 2012. This Decree changes the unique accreditation body model (Instituto Colombiano de Normas Técnicas -Icontec) to the model of several accrediting entities which, in turn, must registered and accredited by The International Society for Quality Assurance in Healthcare (ISQUA), a worldwide reference for accrediting institutions.
According to the mentioned Decree No. 903, the SUA is the set of processes, procedures and tools of voluntary and periodic implementation by the institutions providing health services, health promoting entities, occupational risk management entities and health service providers institutions offering occupational health services, which are intended to verify the gradual compliance of quality levels above mandatory minimum requirements for health care under the direction of the state and the inspection, surveillance and control of the National Superintendence of Health.
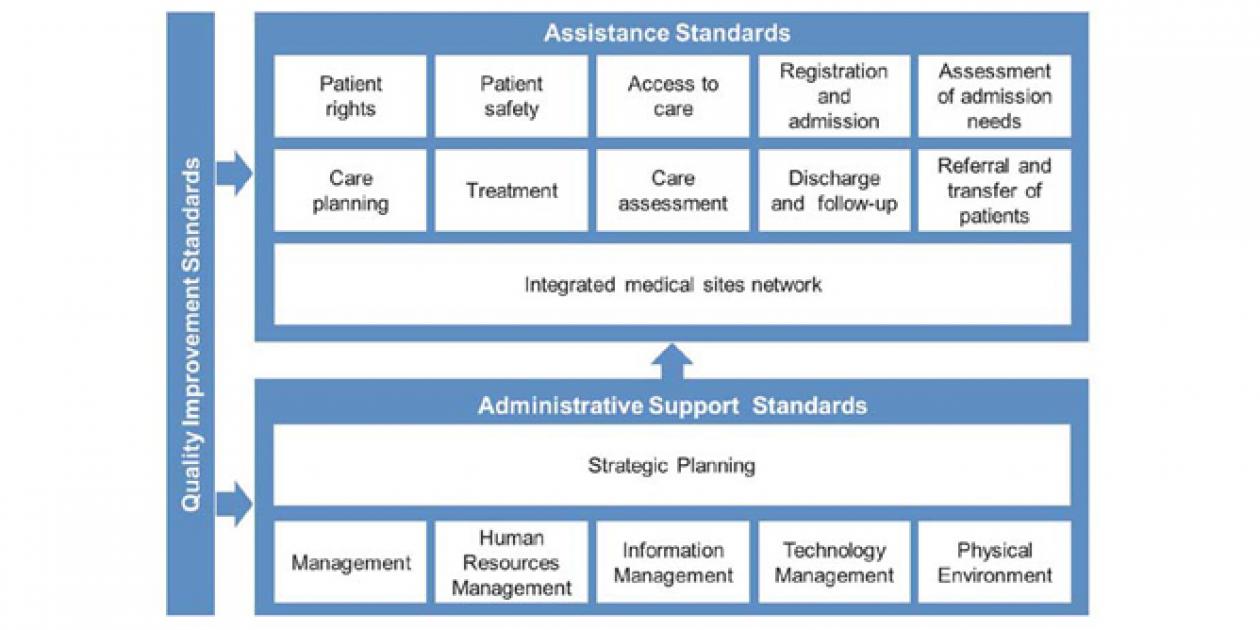
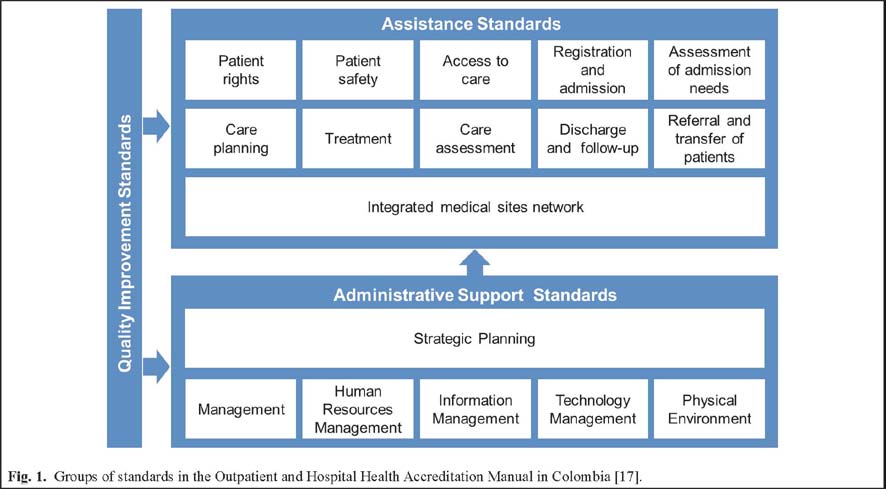
The SUA is based on the Outpatient and Hospital Health Accreditation Manual [17], which applies to the health service provider institutions that offer outpatient, hospital or both services. In this Manual, the 158 standards are arranged as follows: in the first part the Group of Standards for the Assistance Process are set (section 7.1), in the second part appear the Group of Standards for the
Administrative Support to the care processes (sections
7.2 to 7.7) and the third section consisting of five quality improvement standards (section 7.8), which apply to all processes evaluated in both the health care and support to health care standards (Fig. 1).
 [visitante]Para seguir leyendo debe iniciar sesión o darse de alta en el portal[/visitante]
[visitante]Para seguir leyendo debe iniciar sesión o darse de alta en el portal[/visitante]
- The ISO 9001 certification of health institutions
 [visitante]Para seguir leyendo debe iniciar sesión o darse de alta en el portal[/visitante]
[visitante]Para seguir leyendo debe iniciar sesión o darse de alta en el portal[/visitante]
Para seguir leyendo debe iniciar sesión o darse de alta en el portal
Para poder escribir un comentario debe iniciar sesión o darse de alta en el portal.